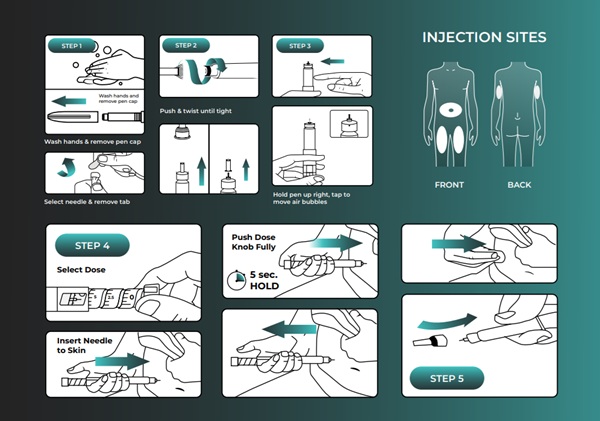
Retatrutide Injection – obesity and type 2 diabetes drug therapy in India.
It is a single molecule that acts as a triple agonist for GLP-1, GIP, and glucagon receptors. It is a powerful “triple-threat” metabolic peptide for weight loss and obesity management.
| Brand Name: | Retatrutide Pen |
| Strength: | Triple Agonist for GLP-1, GIP, and Glucagon Receptors |
| Manufacturer: | Regenmed |
| Price: | On Request |
Retatrutide must always be used under the supervision of a Registered Medical Practitioner (RMP).
Retatrutide is an investigational drug, currently only available through legitimate clinical trials, with Phase 3 trials concluding in 2026.
IPN, New Delhi serves as a facilitator for the procurement of specialized drugs on behalf of patients or treating doctors, enabling access to Retatrutide Injection for personal use. For any queries, including information regarding the cost of the pharmaceutical product in India, please contact the IPN Healthcare Support Team via email or at the following numbers: Mobile/WhatsApp: Mr. Tarun: +91 9891 296 838 | Mr. Neeraj: +91 9811 747 774.
Contact Info
CORPORATE OFFICE:
Basement-B-5-A, Ajit Singh House Building No.12, Yusuf Sarai, South Delhi,
New Delhi:110016 India.
MOBILE / PHONE NUMBER:
M: +91 9811747774, 9891296838.
P / F: +91-11-26532129 / 26536398
EMAIL ID:
Retatrutide is an experimental drug for obesity. It is a triple glucagon hormone receptor agonist (GLP-1, GIP, and GCGR receptors). Retatrutide has been studied in a phase 2 trial involving adults without diabetes but with obesity or preobesity (overweight). Retatrutide is also being evaluated in phase 3 clinical trials. A substudy in adults with type 2 diabetes reported differences in total body fat mass between study groups at 36 weeks.
What are the Side Effects of Retatrutide?
Across clinical studies, the most commonly reported adverse events were gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
Contact IPN – Retatrutide Access in India
For inquiries regarding availability, import process, or cost of Retatrutide Injection :
India Pharma Network (IPN) : Headquartered in New Delhi, with branch offices in Delhi, Gurugram, Mumbai, Bangalore, Kolkata, Chennai, Pune, Ahmedabad, Hyderabad, Chandigarh, Jaipur and Lucknow, IPN supports medicine imports across India on a Named Patient Import Basis and for personal use only. Mobile/WhatsApp: Mr. Tarun: +91 9891 296 838 | Mr. Neeraj: +91 9811 747 774. Email: info@indianpharmanetwork.co.in
NEWS/UPDATES
- FDA approves therapy for rare blood disorder in pediatric patients 12 years and older.
- FDA approved caplacizumab-yhdp.
- FDA approves caplacizumab for pediatric acquired thrombotic thrombocytopenic purpura.
- FDA approves Sanofi’s Cablivi as first therapy for rare blood disorder.
Compliance & Disclaimer
India Pharma Network acts solely as a facilitator for Retatrutide Injection access. IPN does not manufacture, sell, or promote medicines and does not provide medical advice. All imports are:
- Strictly on a Named Patient Import Basis
- Exclusively for personal use
- Not for resale, distribution, or commercial sale
- Subject to applicable Indian laws and regulatory approvals
The prescribing physician remains solely responsible for dosage, therapy, and clinical decisions.
Indian Pharma Network (IPN), New Delhi. India
Indian Pharma Network (IPN) is registered organisation in India, having highly qualified healthcare professionals of pharmaceutical Industry. Specialized in Oncology Products. Helped more than 6,000 patients. Registration Number : 07AAFFI2544E1ZO under GST Act.
Branch Offices in India : New Delhi | Bengaluru | Kolkata | Lucknow | Jaipur | Jodhpur.
Our Overseas Network UK | Russia | Brazil | Mexico | Germany | France| Italy | Spain | Japan | China| Australia | Brazil | Saudi Arabia | South Africa
Mobile / WhatsApp / Phone
M: +91 9811747774, 9891296838.
P: +91-11-26532129 / 26536398
Free Customer Support
info@indianpharmanetwork.co.in
urgent@indianpharmanetwork.co.in