US FDA Approves Venetoclax for Previously Untreated Chronic Lymphocytic Leukemia Patients
Venetoclax is a first-in-class, oral B-cell lymphoma-2 (BCL-2) inhibitor that selectively binds and inhibits the B-cell lymphoma-2 (BCL-2) protein and has been granted five Breakthrough Therapy designations from the FDA. Recently, Venetoclax in combination with obinutuzumab was approved by the U.S. Food and Drug Administration (FDA) for previously untreated patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
The CLL14 Trial was a prospective, multicenter, open-label, randomized Phase 3 CLL14 trial, which was conducted in close collaboration with the German CLL Study Group (DCLLSG). Patients had coexisting medical conditions (total Cumulative Illness Rating Scale [CIRS] score >6 or creatinine clearance <70 mL/min).
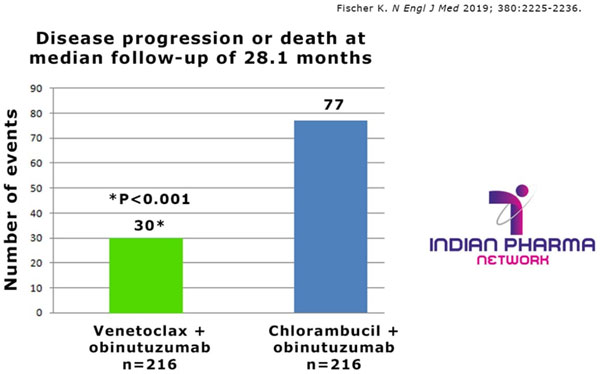
The Real-Time Oncology Review (RTOR) pilot program led to this approval in just over two months after submission of the complete application and the FDA has granted Breakthrough Therapy designation for this combination therapy. Data from the CLL14 trial demonstrated superior progression-free survival in patients when Venetoclax plus obinutuzumab (n=216) was compared to the commonly used standard of care with chlorambucil plus obinutuzumab (n=216) and this formed the basis of their approval.
Venetoclax plus obinutuzumab reduced the risk of progression or death by 67% at a median follow-up of 28 months (range: 0.1 to 36 months) when compared with chlorambucil plus obinutuzumab (hazard ratio: 0.33, 95% confidence interval [CI]: 0.22, 0.51; p<0.0001) even though the median PFS was not reached in either treatment arm. Therapy was completed within 12 months and the majority (87%) of patients did not experience disease worsening with follow up of 28 months
The secondary endpoint was minimal residual disease (MRD) negativity and at three months post treatment completion, Venetoclax plus obinutuzumab demostrated higher rates of MRD when compared to obinutuzumab plus chlorambucil in both bone marrow (57% versus 17%, p<0.0001) and peripheral blood (76% versus 35%, p<0.0001).
Venetoclax is approved in more than 50 countries and is currently under research for use in several other hematologic malignancies including acute myeloid leukemia (AML), multiple myeloma (MM), nonHodgkin lymphoma (NHL) and myelodysplastic syndrome (MDS).
Venetoclax was U.S. FDA approved based on an accelerated approval first, for the treatment of patients with CLL with 17p deletion that have received at least one prior therapy in April 2016 based on their overall response rate. Subsequently, in June 2018, results of the MURANO study led to Venetoclax being approved for the treatment of patients with CLL or SLL who have received at least one prior therapy, with or without 17p deletion. Venetoclax was then approved in combination with azacitidine, or decitabine, or low-dose cytarabine in November 2018 for treating 75 years of age or older adults with newly-diagnosed acute myeloid leukemia (AML), or those that have other medical conditions that don’t permit the use of standard chemotherapy.
This is the fifth Breakthrough Therapy designation for Venetoclax and this milstone also marks the fourth approval for Venetoclax to treat two different types of blood cancer
Reference: U.S. Food and Drug Administration (2018). Approved Drugs:
FDA approves venetoclax in combination for AML in adults. https://www.fda.gov/drugs/fda-approves-venetoclax-combination-amladults.