Neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer
Neratinib is approved by the U.S. Food and Drug Administration for extended adjuvant treatment of early stage HER2-overexpressed/amplified breast cancer adult patients as adjuvant after trastuzumab-based therapy.
The ExteNET trial was a multicenter, randomized, double-blind, placebo-controlled trial of neratinib following adjuvant trastuzumab treatment that evaluated women (n=2,840) with early-stage HER2-positive breast cancer. Patients were randomized to receive either neratinib (n=1420) or placebo (n=1420) for one year within two years of completing adjuvant trastuzumab.
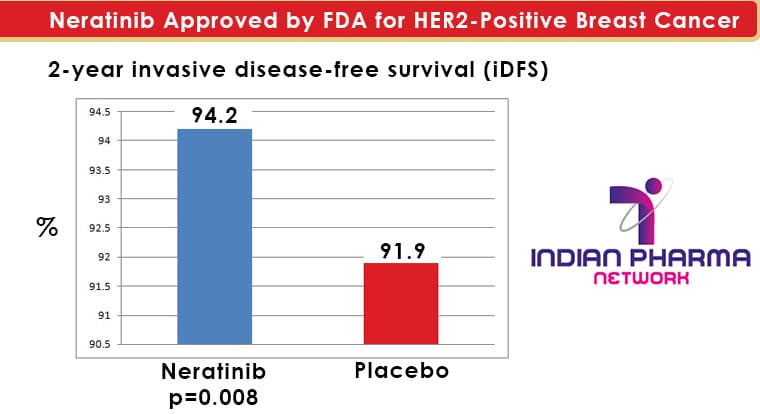
The invasive disease-free survival (iDFS; time between the randomization date to the first occurrence of invasive recurrence (local/regional, ipsilateral or contralateral breast cancer), distant recurrence, or death from any cause, within two years and 28 days of follow-up) was the major efficacy outcome measure. The iDFS was 94.2% in the neratinib group compared to 91.9% in the placebo group (HR 0.66; 95% CI: 0.49, 0.90, p=0.008) after two years.
The recommended dose of neratinib is 240 mg PO OD with food continuously for one year. Diarrhea was seen in 16.8% of neratinib-treated patients and this was the most common adverse reaction leading to discontinuation. As such, antidiarrheal prophylaxis should be started with the first neratinib dose and given during the first 2 cycles (56 days) of treatment and as required thereafter. Drug discontinuation in 1.7% of neratinib-treated patients was also seen due to hepatotoxicity or increases in liver transaminases.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208051s000lbl.pdf
Abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer
Abemaciclib is now US FDA approved for use when combined with an aromatase inhibitor as initial endocrine-based therapy in postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer.
The results of the MONARCH 3, a randomized (2:1), double-blinded, placebo-controlled, multicenter clinical trial in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer was the basis of this approval.
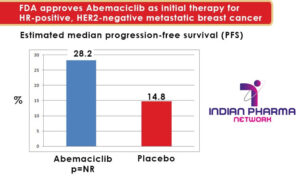
Patients (n= 493) were randomized to abemaciclib 150 mg or placebo, PO BID along with the physician’s choice of either letrozole or anastrozole. 28.2 months (95% CI: 23.5, Not reached) was the estimated median progression-free survival (PFS) (RECIST 1.1) for patients receiving abemaciclib as compared to 14.8 months (95% CI: 11.2, 19.2) for the placebo group (HR 0.540; 95% CI: 0.418, 0.698; p<>). 150 mg PO BID, with or without food is the recommended starting dose of abemaciclib in combination with an aromatase inhibitor.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf